| Attribute | Detail |
|---|---|
| Active Ingredient | Finasteride (1mg) |
| Manufacturer | Cipla Limited |
| Usage | Androgenetic Alopecia (AGA) Treatment |
| Duration of Effect | Continuous use is required for sustained effect |
| Approval | FDA-approved for male pattern hair loss |
Understanding Finpecia
Finpecia, a renowned solution for androgenetic alopecia, is lauded for its effectiveness in halting hair loss and promoting hair regrowth in men. This medication, containing the active ingredient finasteride, targets the root cause of hair thinning by chemically inhibiting the conversion of testosterone to dihydrotestosterone (DHT), a key player in hair follicle miniaturization.
Its manufacturer, Cipla Limited, a leading pharmaceutical company, guarantees the quality and efficacy of Finpecia. The product's success is attributed to its targeted approach, offering hope to many who struggle with hair loss, thereby improving their quality of life and self-esteem.
What is Finpecia?
Finpecia is a prescription medication designed specifically for the treatment of male pattern baldness. By leveraging the active ingredient finasteride, it slows hair loss and, in some cases, reverses it, making it a cornerstone treatment for managing androgenetic alopecia.
How does Finpecia work?
Finpecia operates by inhibiting the 5-alpha-reductase enzyme, responsible for the conversion of testosterone to DHT. Reducing DHT levels in the scalp halts the progression of hair loss and facilitates the regrowth of hair, offering a scientific approach to combating baldness.
The origin and manufacturing company of Finpecia (Cipla Limited)
Cipla Limited, a global pharmaceutical giant based in India, manufactures Finpecia. Known for its rigorous quality control and innovative healthcare solutions, Cipla has established itself as a trustworthy source of effective medications, including Finpecia, for various health conditions.
Analyzing the Risk and Safety of Finpecia
While Finpecia is celebrated for its efficacy, it's crucial to consider its safety profile and potential risks. Clinical studies and user experiences underline the importance of understanding the possible side effects, which range from mild to severe, and the necessity of consulting healthcare professionals before commencing treatment.
Counterfeit medications pose a significant risk, undermining treatment effectiveness and patient safety. Distinguishing genuine Finpecia from counterfeit versions is essential, highlighting the importance of purchasing from reputable sources and recognizing the hallmarks of authenticity provided by Cipla Limited.
Known dangers of Finpecia
Despite its benefits, Finpecia can cause side effects such as sexual dysfunction, depression, and allergic reactions in a minority of users. These risks necessitate a thorough evaluation by healthcare providers to ensure patient safety and well-being.
How to differentiate genuine Finpecia from counterfeit versions
To ensure authenticity, genuine Finpecia features distinct packaging and branding by Cipla Limited, along with a hologram and batch numbers that can be verified for authenticity. Purchasing from accredited pharmacies minimizes the risk of encountering counterfeit products.
Risk cases from counterfeit usage
Counterfeit Finpecia, lacking the correct active ingredient or containing harmful substances, poses serious health risks, including ineffective treatment and adverse reactions. Identifying and reporting counterfeit products is critical for community safety.
Demystifying the Use of Finpecia
Proper usage of Finpecia, including adherence to dosage and administration guidelines, is paramount for achieving optimal results. Understanding when and how to take the medication, as well as recognizing situations where its use is contraindicated, ensures both efficacy and safety for users.
Being informed about the potential side effects and their management strategies allows patients to navigate their treatment with confidence, making informed decisions regarding their health and treatment options.
The correct dosage and method of taking Finpecia
The recommended dosage of Finpecia is one 1mg tablet daily, with or without food. Consistency in timing enhances the medication's effectiveness, and long-term use is often necessary to maintain hair regrowth and prevent further hair loss.
Cases when Finpecia should not be taken
Finpecia is contraindicated in women, especially during pregnancy, due to the risk of birth defects, and in individuals with liver disease or allergic reactions to finasteride. A medical consultation is essential to assess suitability for treatment.
Possible side effects and how to manage them
While most side effects are mild and reversible, including decreased libido and ejaculation issues, managing them involves consultation with a healthcare provider. Discontinuation or adjustment of dosage may be recommended based on individual responses.
Finpecia Versus Other AGA Treatment Drugs
Finpecia stands out in the crowded field of AGA treatments for its targeted mechanism of action and proven efficacy. Comparing it with other medications like Propecia, Minoxidil, and Dutasteride offers insight into its unique advantages and suitability for different patients.
Understanding the interaction between Finpecia and other AGA drugs is crucial for developing a comprehensive treatment plan, considering the synergistic effects and potential for enhanced outcomes when used in combination.
Comparison of Finpecia and Propecia (finasteride generic)
While Finpecia and Propecia both contain finasteride, their difference lies in branding and pricing. Both are effective in treating AGA, but Finpecia is often preferred for its cost-effectiveness and accessibility.
Table: Comparative Analysis of Finpecia, Propecia, Minoxidil, and Dutasteride
| Medication | Mechanism of Action | Usage | Common Side Effects |
|---|---|---|---|
| Finpecia (Finasteride 1mg) | Inhibits DHT production | Daily oral intake | Sexual dysfunction, mood changes |
| Propecia (Finasteride 1mg) | Inhibits DHT production | Daily oral intake | Sexual dysfunction, mood changes |
| Minoxidil | Stimulates hair growth | Topical application | Scalp irritation, hair growth on face |
| Dutasteride | Inhibits DHT production more potently than finasteride | Daily oral intake | Sexual dysfunction, mood changes |
The Indian Paradox: Availability of Finpecia despite Active Patents
The availability of Finpecia in India, despite the presence of active patents, underscores the complexities of the pharmaceutical patent laws in the country. This scenario highlights the balancing act between encouraging innovation and ensuring accessibility to essential medications for the population.
Understanding the legal framework and the strategic maneuvers by companies like Cipla Limited to provide crucial medications like Finpecia within this context is essential. It reflects on the broader challenges and opportunities in the global pharmaceutical industry.
Understanding the Indian pharmaceutical patent law
Indian pharmaceutical patent law is designed to balance patent protection with the need to provide affordable medication. It allows for certain flexibilities, such as compulsory licensing and provisions for generic drug production, facilitating the availability of medications like Finpecia.
How Finpecia bypassed patent laws
Cipla Limited's ability to manufacture and distribute Finpecia in India, despite patent laws, is attributed to the legal provisions that prioritize public health needs over patent rights. This approach has enabled wider access to affordable treatment options for AGA.
Addressing Common Queries about Finpecia
As the use of Finpecia grows, so do the questions and concerns from potential users. Addressing these queries is crucial for informed decision-making, ensuring that individuals have accurate information regarding the medication's composition, regulatory approval status, and its benefits and drawbacks.
Providing clear, evidence-based answers helps demystify the treatment, empowering patients with the knowledge needed to navigate their health choices effectively.
Does Finpecia contain Quinoline Yellow?
No, Finpecia does not contain Quinoline Yellow. The active ingredient is finasteride, and the medication's excipients do not include this colorant, ensuring its safety and efficacy for users.
Is the use of Finpecia approved in Japan?
As of the latest updates, Finpecia itself may not be specifically approved in Japan; however, finasteride, the active ingredient in Finpecia, is approved for use in treating male pattern baldness under different brand names.
FAQs Finpecia
1. What is Finpecia used for?
Finpecia is a medication primarily used to treat male pattern hair loss, a condition medically known as androgenetic alopecia. It helps to prevent further hair loss and promote hair regrowth in men.
2. How does Finpecia work?
Finpecia contains the active ingredient finasteride, which works by inhibiting the enzyme 5-alpha reductase. This enzyme converts testosterone into dihydrotestosterone (DHT), a hormone that can shrink hair follicles and lead to hair loss in men. By blocking the conversion of testosterone to DHT, Finpecia helps to maintain hair follicle size and prevent hair loss.
3. Is Finpecia suitable for women?
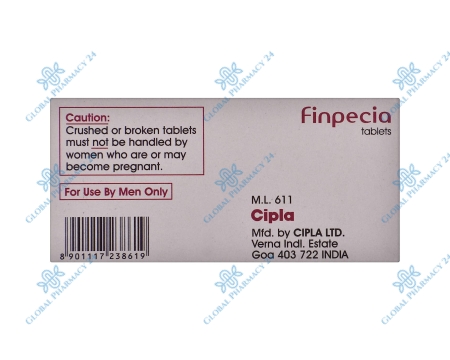
No, Finpecia is not recommended for use in women, especially pregnant women or those planning to become pregnant. Finasteride, the active ingredient in Finpecia, can cause birth defects in a developing male fetus. Women who are pregnant or may become pregnant should avoid handling crushed or broken tablets of Finpecia.
4. What are the common side effects of Finpecia?
Common side effects of Finpecia may include decreased libido, erectile dysfunction, ejaculation disorder, breast tenderness or enlargement, and rash. These side effects are usually mild and may resolve with continued use or upon discontinuation of the medication. However, if you experience any severe or persistent side effects, it's important to consult your healthcare provider.